Background: Anaplastic large cell lymphomas (ALCLs) are CD30-positive T-cell lymphomas that share pathologic features but differ in clinical presentation, outcome, and molecular features. The World Health Organization (WHO) and International Consensus Classification (ICC) classify ALCLs by presence or absence of ALK rearrangements (R) and clinical presentation (systemic, cutaneous [c], or breast implant-associated [BIA]). ICC, but not WHO, recognizes DUSP22-R as defining a new genetic subtype of ALK- ALCL. The classifications otherwise do not reflect additional molecular heterogeneity in genetics (e.g., TP63-R) or therapeutic vulnerabilities (e.g., JAK-STAT3 pathway activation).
Methods: ALCLs (N=689) underwent expert consensus review (WHO/ICC) through the Lymphoma/Leukemia Molecular Profiling Project (LLMPP). All cases also underwent genetic subtyping (ALK, DUSP22, TP63, and triple-negative [TN]) using fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC), as well as IHC for phospho-STAT3 Tyr705 (pSTAT3). RNAseq was performed and evaluable in 393 cases; the remaining cases had insufficient tissue, tumor content, RNA yield or quality, and/or sequencing data quality. Sequenced and non-sequenced sub-cohorts had similar demographics and subtype distribution.
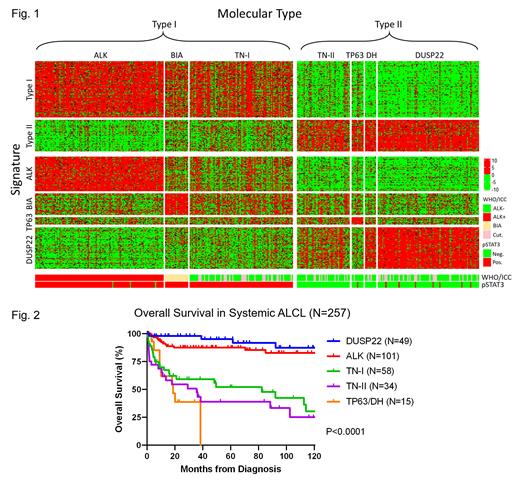
Results: Unsupervised gene expression profiling (GEP) identified 2 main molecular types of ALCL. Type I ALCLs predominantly included ALK+ ALCLs, BIA-ALCLs, and a subset of TN ALCLs (designated TN-I), whereas Type II ALCLs predominantly included ALCLs with DUSP22-R, TP63-R, or both (double-hit; DH), and the remaining TN ALCLs (TN-II). Type I ALCLs were strongly associated with pSTAT3 expression (74.2±26.4% positive malignant cells vs 9.9±22.7% for Type II; P<0.0001). An independently derived pSTAT3 staining threshold of 30% assigned Type I vs II with 91% accuracy. A third cluster of ALCLs, mostly cALCL, showed an epithelial GEP signature rather than a lymphoma signature; these cases were assigned to Types I or II based on pSTAT3 IHC. Distinct sub-signatures were identified for ALK+, DUSP22-R, TP63-R, and BIA ALCL (Fig. 1), but not ALK- ALCL or cALCL.
Gene set enrichment analysis showed Type I ALCLs to be enriched for JAK-STAT3 signaling genes (normalized enrichment score [NES], 2.22; FDR<0.0001) and related pathways, such as TNFα-NFκB signaling (NES, 2.21; FDR<0.0001). In contrast, Type II ALCLs were enriched for cell cycle, DNA repair, epigenetic, and metabolic pathway genes, but not for major tyrosine kinase-mediated signaling pathway genes. Enriched epigenetic pathways included chromatin modifying enzymes (NES, -1.86; FDR=0.002) and histone methylation (NES, -1.71; FDR=0.002). EZH2 was the most overexpressed gene in Type II ALCLs (fold-change, 7.74; FDR=8.84×10 -306). At the protein level, EZH2 IHC H-scores were 275±45 in Type II and 168±70 in Type I ALCLs (P<0.0001); H3K27me3 H-scores were 170±81 and 76±63, respectively (P<0.001). The top metabolic gene set involved cholesterol biosynthesis (NES, -1.88; FDR=0.002).
Overall survival (OS) data were available in 257 systemic ALCL patients (145 sequenced and 112 non-sequenced; cALCL and BIA-ALCL were excluded). Non-sequenced TN ALCLs were assigned to TN-I or TN-II using pSTAT3 IHC. The results supported earlier data indicating favorable prognosis of DUSP22-R ALCL (5 y OS, 95%) and ALK+ ALCL (87%), intermediate prognosis of TN ALCL (TN-I, 52% and TN-II, 38%; P=NS), and poor prognosis of TP63-R/DH ALCL (0%)(P<0.0001; Fig. 2).
Conclusions: Two overarching molecular types of ALCL exist, predominantly associated with the presence (Type I) or absence (Type II) of the JAK-STAT3 signaling program. Distinct GEP signatures exist for ALK+ ALCL and BIA-ALCL (predominantly Type I), and DUSP22-R ALCL and TP63-R ALCL (predominantly Type II). TN ALCLs lacking ALK-R, DUSP22-R, and TP63-R can be stratified into TN-I and TN-II subtypes. pSTAT3 IHC has >90% accuracy as a surrogate for GEP-based subtyping. ALK- ALCL and cALCL cluster by molecular subtype rather than by defining GEP signatures. Type II ALCLs are enriched for targetable epigenetic and metabolic pathways, including EZH2/histone methylation and cholesterol biosynthesis. This molecular classification is diagnostically, prognostically, and potentially therapeutically relevant, and can be applied using FISH and IHC in routine practice and in the clinical trial setting.
Disclosures
Rimsza:Roche: Other: Consulting; NanoString: Other: Licensed intellectual property. Scott:Janssen and Roche: Research Funding; Abbvie, AstraZeneca, Incyte: Consultancy. Savage:Merck: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria; Roche: Research Funding; Seagen: Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Yoshino:Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin: Honoraria. Kahl:Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; ADCT: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria. de Leval:Lunaphore: Consultancy; Novartis: Consultancy; Bio Ascend: Consultancy; Bayer: Consultancy; Abb Vie: Consultancy. Cerhan:Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Protagonist: Other: Safety Monitoring Committee; NanoString: Research Funding; Genentech: Research Funding. Ansell:Regeneron Pharmaceuticals Inc: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Affirmed: Other: Contracted Research; ADC Therapeutics: Other: Contracted Research; Seagen Inc: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal